Duloxetine Recall: FDA Warns Of Increased Suicide Risk
Editor's Notes: "Duloxetine Recall: FDA Warns Of Increased Suicide Risk" have published on January 23, 2012. Because of the importance of this issue, we’ve analyzed the latest information so that you can make your own educated decisions.
Our expert team has put together this guide to help you get up-to-speed on everything you need to know about "Duloxetine Recall: FDA Warns Of Increased Suicide Risk".
FAQ
Have there been any new developments regarding the Duloxetine recall? The U.S. Food and Drug Administration (FDA) recently issued a warning about the increased risk of suicide associated with the use of Duloxetine, a widely prescribed antidepressant.

Risks behind bars: Suicide attempts have increased at county jail - Source www.observertoday.com
Question 1: What is Duloxetine?
Duloxetine is an antidepressant medication commonly used to treat conditions such as depression, anxiety, and chronic pain. It belongs to a class of medications known as selective serotonin and norepinephrine reuptake inhibitors (SNRIs).
Question 2: Why did the FDA issue the warning?
The FDA warning is based on a review of data from clinical trials involving patients taking Duloxetine. The review found that patients taking Duloxetine had a slightly increased risk of suicidal thoughts and behaviors compared to patients taking a placebo.
Question 3: Who is at risk?
The increased risk of suicide associated with Duloxetine is highest in children, adolescents, and young adults.
Question 4: What should I do if I am taking Duloxetine?
If you are currently taking Duloxetine, it is important to talk to your doctor about the potential risks and benefits of continuing treatment. Do not stop taking Duloxetine without first consulting with your doctor.
Question 5: What are the symptoms of suicidal thoughts and behaviors?
Symptoms of suicidal thoughts and behaviors can include talking about wanting to die, feeling hopeless or worthless, withdrawing from social activities, and having difficulty sleeping or concentrating.
Question 6: Where can I get help?
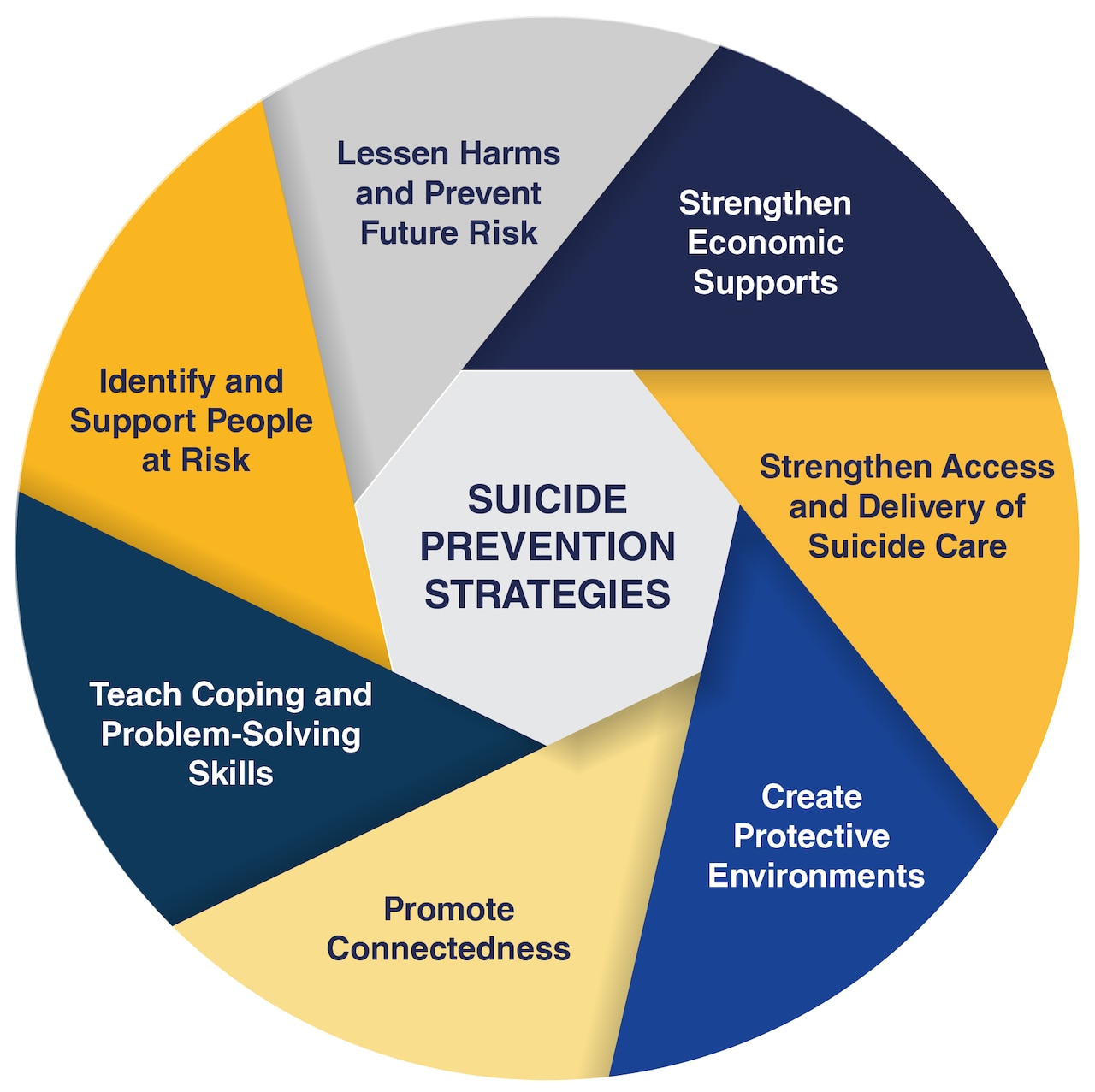
If you are experiencing suicidal thoughts or behaviors, it is important to seek help immediately. You can call the National Suicide Prevention Lifeline at 1-800-273-8255 or visit their website at https://suicidepreventionlifeline.org for more information and resources.
Remember: Suicide is a serious issue, and it is important to seek help if you or someone you know is struggling with suicidal thoughts or behaviors.
Tips
The U.S. Food and Drug Administration (FDA) has issued a warning about the increased risk of suicide associated with the antidepressant duloxetine (Cymbalta). This warning is based on a review of clinical trials that found that people taking duloxetine were more likely to experience suicidal thoughts and behaviors than those taking a placebo. The FDA recommends that healthcare professionals monitor patients taking duloxetine closely for any signs of suicidal ideation or behavior.
Tip 1: Be aware of the risks.
Before taking duloxetine, talk to your doctor about the risks of suicide. Make sure you understand the symptoms of suicidal ideation and behavior, and know what to do if you experience any of these symptoms.
Tip 2: Monitor yourself closely.
If you are taking duloxetine, pay attention to your thoughts and feelings. If you notice any changes in your mood or behavior, such as feeling more depressed, anxious, or irritable, or if you have thoughts of suicide, contact your doctor immediately.
Tip 3: Tell your family and friends.
Let your family and friends know that you are taking duloxetine and about the risks of suicide. Ask them to keep an eye on you and to report any changes in your mood or behavior to your doctor.
Tip 4: Seek professional help.
If you are experiencing suicidal thoughts or behaviors, seek professional help immediately. There are many resources available to help you, including crisis hotlines, mental health professionals, and support groups.
Tip 5: Do not stop taking duloxetine abruptly.
If you need to stop taking duloxetine, do not stop suddenly. Stopping duloxetine abruptly can cause serious side effects, including seizures.
Summary:
The FDA warning about the increased risk of suicide associated with duloxetine is a serious reminder of the importance of monitoring patients taking this medication closely for any signs of suicidal ideation or behavior. Healthcare professionals should be aware of the risks and should monitor patients accordingly. Patients taking duloxetine should be aware of the risks and should monitor themselves closely for any changes in mood or behavior. If you are experiencing suicidal thoughts or behaviors, seek professional help immediately.
Transition to the article's conclusion:
The FDA is continuing to review the safety of duloxetine and will provide updates as new information becomes available. In the meantime, healthcare professionals and patients should be aware of the risks and should take appropriate precautions.
Duloxetine Recall: FDA Warns Of Increased Suicide Risk
Antidepressant drug Duloxetine has been recalled due to increased risk of suicide.
- Medication: Duloxetine, sold under the brand name Cymbalta, is an antidepressant medication commonly prescribed to treat depression, anxiety, and chronic pain.
- Risk: The Food and Drug Administration (FDA) has warned of an increased risk of suicidal thoughts and behaviors in patients taking Duloxetine, particularly in children and young adults.
- Recall: The FDA has ordered a recall of all extended-release capsules of Duloxetine in the United States.
- Symptoms: Patients taking Duloxetine should be aware of the potential side effects, including thoughts of self-harm or suicide, agitation, and insomnia.
- Monitoring: Healthcare professionals are advised to closely monitor patients taking Duloxetine and consider alternative treatment options.
- Alternatives: There are other antidepressants available that may be safer for patients at risk of suicide, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
This recall highlights the importance of being aware of the potential risks associated with medications, especially those used to treat mental health conditions. Patients should always consult with their healthcare provider to discuss the benefits and risks of any medication they are taking.
DOD Takes Public Health Approach to Suicides > U.S. Department of - Source www.defense.gov
Duloxetine Recall: FDA Warns Of Increased Suicide Risk
The Food and Drug Administration (FDA) has issued a warning about the increased risk of suicide associated with the use of duloxetine, an antidepressant medication. The FDA's warning comes after a review of several studies that found that people taking duloxetine were more likely to experience suicidal thoughts and behaviors than people taking a placebo.

Entenda a importância da certificação FDA para materiais em contato com - Source vedacoesmakita.com.br
Duloxetine is a selective serotonin and norepinephrine reuptake inhibitor (SNRI), a type of antidepressant that is used to treat depression, anxiety, and chronic pain. The FDA's warning applies to all forms of duloxetine, including capsules, tablets, and extended-release capsules.
The FDA is advising doctors to carefully monitor patients taking duloxetine for signs of suicidal thoughts and behaviors. The FDA is also advising patients taking duloxetine to be aware of the potential for suicidal thoughts and behaviors and to seek help immediately if they experience any of these symptoms.
The FDA's warning is a serious reminder of the importance of mental health awareness. Depression is a serious mental illness that can lead to suicide. If you or someone you know is struggling with depression, please seek help immediately. There are many resources available to help people with depression, and there is hope for recovery.
Conclusion
The FDA's warning about duloxetine is a reminder that all medications have the potential for side effects. It is important to be aware of the risks and benefits of any medication you are taking, and to talk to your doctor about any concerns you have. If you are taking duloxetine, it is important to be aware of the potential for suicidal thoughts and behaviors, and to seek help immediately if you experience any of these symptoms.
The FDA's warning is also a reminder of the importance of mental health awareness. Depression is a serious mental illness that can lead to suicide. If you or someone you know is struggling with depression, please seek help immediately. There are many resources available to help people with depression, and there is hope for recovery.